1. Write the full form of IUPAC.
- The full form of IUPAC is International Union of Pure Applied Chemistry.
2. write about saturated and unsaturated hydrocarbons.
- Hydrocarbon with a single covalent bond between the carbon atoms are called saturated hydrocarbons. They are also called alkanes and paraffin which are less reactive towards chemical reaction. For eg. Methane (CH4), ethane (C2H6) etc.
- Hydrocarbons with double or triple covalent bond between carbon atoms are called unsaturated hydrocarbons. They are of two types alkenes (having double bond between carbons) and alkynes (having triple bond between carbons).
Eg. of alkene- Ethene (C2H4), propene (C3H6) etc.
Eg. of alkyne- Ethyne (C2H2), propene (C3H4) etc.
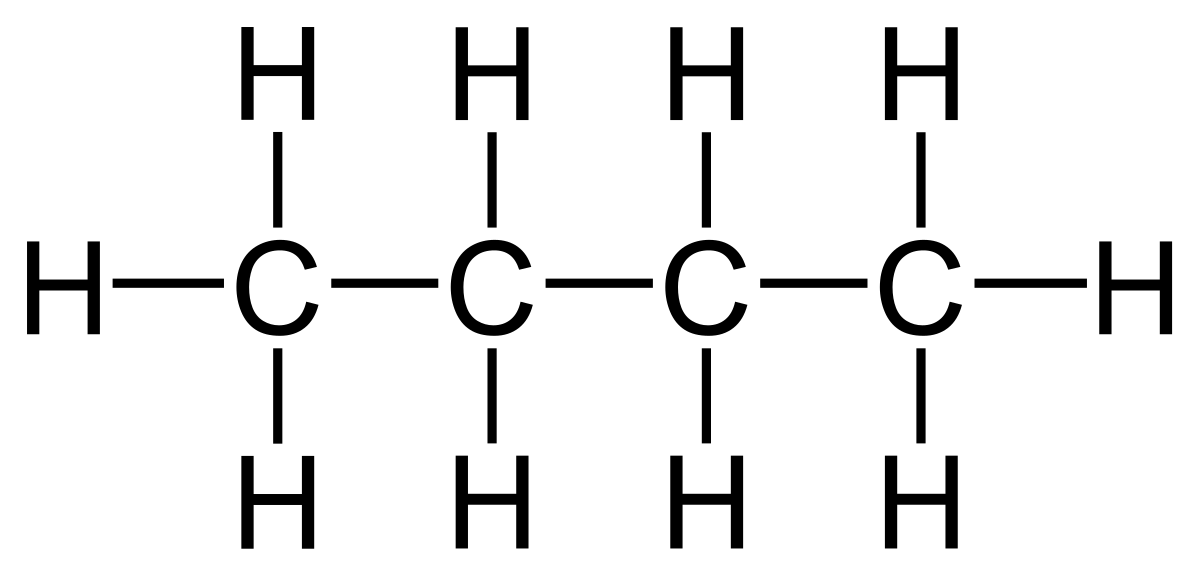
3. Write the structural formula of butane, propane and acetylene.
BUTANE
PROPANE
ACETYLENE (ETHYNE)

No comments:
Post a Comment